Test Announcement for COVID-19
CLIA certified laboratory that utilizes advanced RT-PCR based platforms.
Unrivaled precision and care
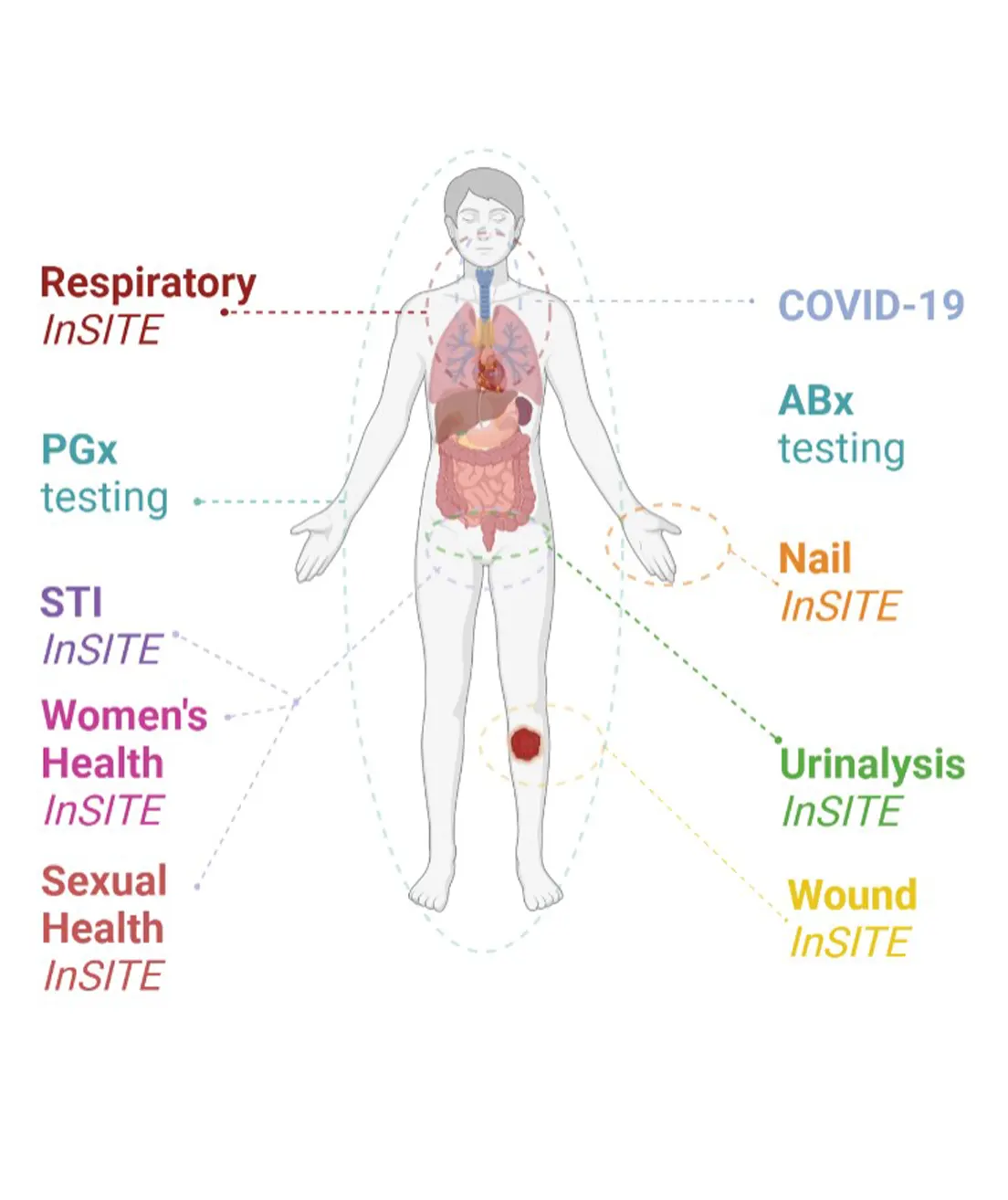
- Find out whether a certain medicine could be effective for you
- Find out what the best dosage might be for you
- Predict whether you will have a serious side effect from a medicine

The Prism Approach
Prism Molecular utilizes real-time PCR technology which is a very sensitive and accurate technique for detecting pathogens and antibiotic resistance gene markers. This technique involves a single step detection method by rapidly amplifying a specific target segment of the pathogenʼs DNA/RNA. In each PCR reaction, the unique target DNA fragment is amplified into billions of copies that results in pathogen and antibiotic resistance marker gene recognition.
Accessibility is a key priority
Prism Molecular is dedicated to providing accurate, actionable, accessible, and timely results for infectious disease testing. The company offers a range of infectious disease testing services, including COVID-19 testing, and uses advanced technologies to provide reliable results.
Accuracy is a top priority for Prism Molecular, and the company has implemented stringent quality control measures to ensure that its results are highly accurate. The company’s testing processes are designed to minimize the risk of false positives and false negatives, and its staff are highly trained in handling and analyzing samples.
Prism Molecular also recognizes the importance of providing actionable results that can be used to inform clinical decision-making. The company’s testing reports are designed to be easy to understand and interpret, and its staff are available to provide guidance and support to healthcare providers and patients as needed.
COVID-19 pandemic has impacted the world in significant ways
Worldwide Statistics
Credentials


